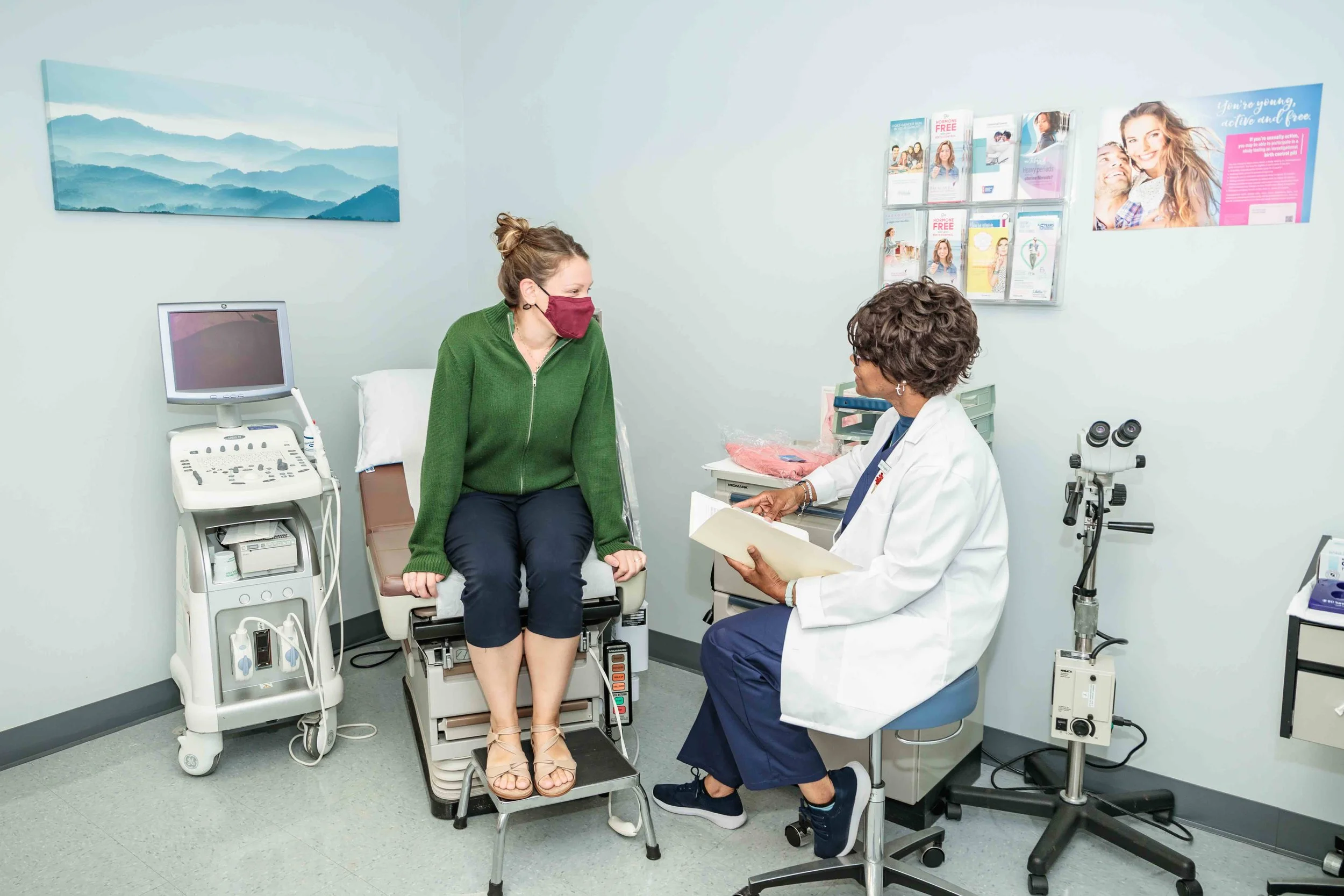
The CRP legacy
CRP is a dedicated Clinical Research site serving the Philadelphia community for over 25 years.
CRP works hard to bring revolutionary new products and treatments to market through high-quality research, all while remaining focused on the one thing that matters most to us: Participant care.
Patient-centric, sponsor-aligned
Focus on diversity
Our staff is diverse, just like the community and study participants we serve. We foster a culture that embraces diversity in all forms.
Embracing technology
Our fully electronic Clinical Trial system allows us to minimize administrative burden and streamline operations so our team can focus more on patient engagement and protocol adherence.
Patient centricity
Our participants are the reason we come to work everyday. Our decisions and daily processes are guided by the principal that participants will always be the heart of our work.
Clinical expertise
We are proud of our committed research team, most of whom have been with our site for over 15 years. Our cohesiveness as a team and our collective experiences enable us to develop innovative solutions for the complex needs of our clinical trials.
What sets us apart
8,000+
participants from the Philadelphia community since 1996
300
successfully completed clinical trials, across a range of therapeutic areas.
80 yrs
of combined experience from our research staff, who have been with CRP for over 2 decades
A fully electronic Clinical Trial Management System, with remote monitoring capabilities, allows us time to focus on participants.
Our state-of-the-art research facility has three exam rooms and dedicated coordinator offices for the comfort of our participants
Meet the experts
-

Aneesh Vaze
Director of Clinical Operations
MBAWith 15 years of pharmaceutical industry experience, Aneesh has been leading CRP since 2020
-

Eugene Andruczyk
Principal Investigator
MBA, DOBoard-certified in Obstetrics/Gynecology, Eugene has been with CRP since 2006
-

Liz Usmiani
Office Manager
Liz is responsible for HR, invoicing, social media & patient recruitment, and keeping CRP on its toes. She has been with CRP since 2020
-

Lolita Vaughan
Sub-Investigator
CNRP, CCRCA certified Women’s Health Nurse Practitioner for over 23 years, Lolita has been with CRP since 2005
-

Donna Gavarone
Investigator
DOA Board-certified Internal Medicine investigator, Donna has worked with CRP since 2021
-

Ivette Vazquez
Quality Assurance Manager
RMA, CCRCA trained medical assistant and CCRC certified, Ivette has worked with CRP since 2002
-

Stacey Scott
Coordinator
Stacey has been with CRP since 2023
-

Theresa Listner
Coordinator
Theresa is a seasoned coordinator with over 16 years of experience in clinical research. Theresa has been with CRP since 2023
-

Ruby Pereira
Coordinator
Ruby has been with CRP since 2002